|
|
|
An active ingredient and impurities are not separated enough. |
|
The active imgredient and impurities are still not separated enough. |
|
Establishment of the measurement condition that completely separates the active imgredient and impurities. |
|
|
Understanding the impurities. |
|
Optimization of reaction condition and refining process based on information about the impurities. |
|
Permanent producing of high-quality APIs. |
|
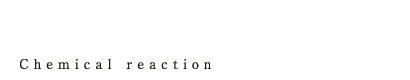
| We perform various organic synthesis reactions including many kinds of condensation reactions by making use of not only general GL or SUS reactors but also autoclaves that are capable of pressurization up to 1 MPa, reactors that are capable of heating up to 230 degree Celsius and high corrosion resistance reactors. In order to produce high quality medicines safely, quickly and environmental friendly, we devise the means of aftertreatment or reaction conditions, not just by performing reaction. |
|
 |
 |

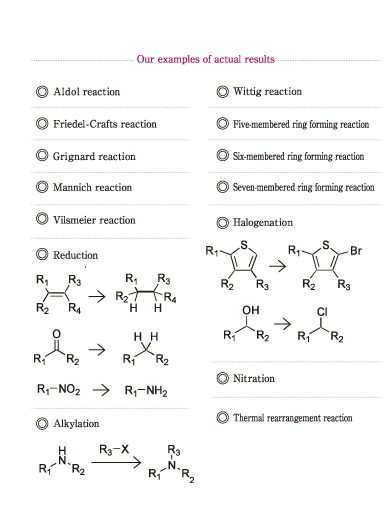
To increase crystal purity, there are variety of ways. For example, the cleaning process in the reaction or modifying the crystallization itself.
On the other hand, some medicines exhibit ''crystal polymorphism'',
for example, some may have a different melting point, or another bulk density, solubility and thermodynamic stability, even though they each have same structure. In our company, in order to obtain the crystal form you require, we gather information regarding the crystallization conditions and we have been producing medicines in actual scale. |
|
 |
|